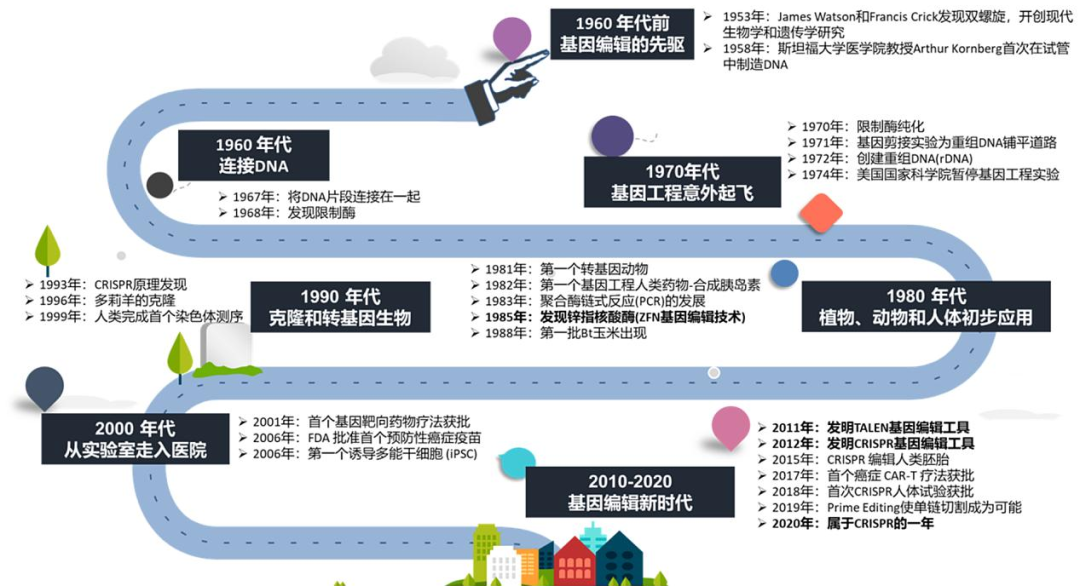
经过激烈的投票
春季PF赛事的辩题确定为基因编辑
面对如此深奥的话题和大量的医学术语
不免让人感到难以下手
因此,春季PF辩题解析系列
将从基因编辑的基础知识与发展历程切入
循序渐进地带领大家分析辩题
今天就为大家带来
春季PF辩题解析①
希望能帮助大家更好地了解基因编辑
为赛事做好充分的准备~

近年来,基因编辑技术频频成为学术界和公众关注的焦点。自2012年CRISPR技术在细胞基因编辑领域取得突破性进展,相关研究成果首次发表于《Science》杂志以来,该领域便进入了高速发展阶段。
2020年,CRISPR技术荣获诺贝尔奖,标志着其科学价值得到国际权威认可。2023年,全球首款CRISPR疗法获批上市,为遗传病的治愈带来了新的希望。与此同时,中国科学家在碱基编辑领域取得的最新突破,进一步提升了公众对基因编辑技术的期待。
但首先,我们先来看看---基因编辑是什么?
让我们直接看看两位专家是如何通俗易懂的解释基因编辑的:
来自中国科学院遗传与发育生物学研究所基因组编辑中心主任,高彩霞说:基因编辑能够精准、快速地改造生命体,它是一项改变生命科学及应用范式的颠覆性技术。它是两个模块:第一个模块就像GPS或者叫搜索功能,在一段很长的文字中,迅速定位要找的那个字。接着,另一个模块编辑模块,用橡皮擦改、铅笔写,是非常精准的。
Gao Caixia, director of the Genome Editing Center at the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences, said: "Gene editing can accurately and quickly modify living organisms, and it is a disruptive technology that changes the paradigm of life science and application. It's two modules: the first module acts like a GPS or search function that quickly locates the word you're looking for in a long paragraph of text. Then, another module editing module, with erasing, pencil writing, is very accurate.
来自北京大学基因组编辑研究中心主任、昌平实验室领衔科学家,魏文胜说:所有的生命细胞都是被编码的,它最底层的遗传信息是由“ATCG”四个字母组成的。我们编辑的是它的排列组合,比如擦除、替换、增加、删除。
Wei Wensheng, director of the Genome Editing Research Center at Peking University and lead scientist in Changping's lab, said: "All living cells are encoded, and its lowest level of genetic information is composed of four letters" ATCG ". We edit its permutations, such as erase, replace, add, delete.
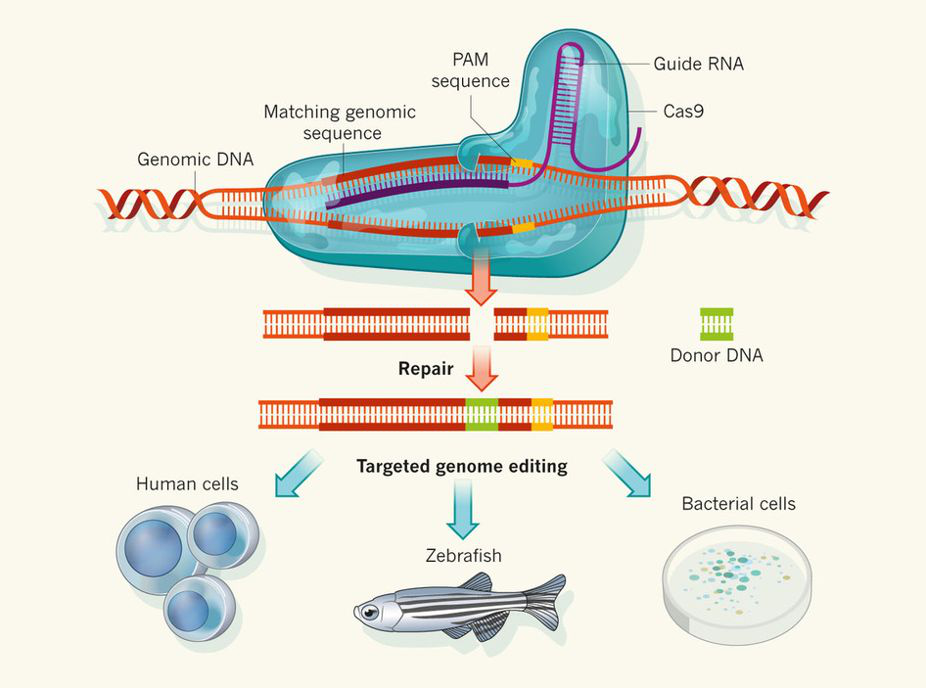
CRISPR/Cas系统介导的基因编辑技术
出现时间:2012年CRISPR/Cas系统是细菌和古细菌中进化出来用于抵御噬菌体及外源DNA入侵的适应性免疫系统,通过CRISPR RNA (crRNA)和trans-activating crRNA (tracrRNA)以及Cas9蛋白组成的复合体抵御外源性DNA的入侵。
Time of appearance: The 2012 CRISPR/Cas system is an adaptive immune system that evolved in bacteria and archaea to defend against phages and foreign DNA invasion, The invasion of exogenous DNA is defended against by complexes consisting of CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA), as well as Cas9 proteins.
由于该技术较前两代技术设计和制备更加简单,成本更低,编辑效率更高,CRISPR/Cas自诞生以来,迅速发展,已经成为生命科学领域最耀眼、最有前景的技术。
连续多年被《Nature》评为最有前景的技术之一,2015年被《Science》评为技术突破第一名,2014、2016年被《麻省理工科技评论》评为10项突破技术之一,2020年诺贝尔化学奖。
Because the technology is simpler to design and prepare than the previous two generations of technology, the cost is lower, and the editing efficiency is higher, CRISPR/Cas has developed rapidly since its birth, and has become the most dazzling and promising technology in the field of life science. It has been rated as one of the most promising technologies by Nature for many years, the first technological breakthrough by Science in 2015, one of the 10 breakthrough technologies by MIT Technology Review in 2014 and 2016, and the Nobel Prize in Chemistry in 2020.
近几年CRISPR/Cas基因编辑技术飞速发展,推广应用到了生物、医学、农业以及环境等多个领域,造就了一批批科研奇迹,尤其是在遗传病的治疗、疾病相关基因的筛查与检测、肿瘤治疗以及动植物的改造、病原微生物防治等领域有着巨大的潜力,也将深远地影响整个世界。
In recent years, CRISPR/Cas gene editing technology has developed rapidly and been applied to many fields such as biology, medicine, agriculture and the environment, creating batches of scientific research miracles, especially in the treatment of genetic diseases, the screening and detection of disease-related genes, tumor treatment, the modification of animals and plants, and the prevention and treatment of pathogenic microorganisms. It will have a profound impact on the entire world.
结语
基因组编辑技术目前发展迅速,主要依靠CRISPR-Cas系统的RNA引导工具,这些工具让我们能够更精确、更容易地修改基因。未来,这些技术将在特异性、效率和传递方式上继续改进。研究重点包括开发更精准的递送载体、减少脱靶效应,以及更好地控制编辑结果,从而提高编辑的准确性。核酸引导系统可能仍然是基因组编辑的核心,因为它们易于编程和适应。
不过,编辑方式可能会转向更短暂的方法,以减少长期基因变化的风险或降低免疫反应。人工智能(AI)的进步将帮助我们模拟复杂的编辑场景,预测编辑结果,并设计更安全的编辑器,加速安全治疗方法的开发。
Genome editing technology is advancing rapidly and relies on the CRISPR-Cas system's RNA-guided tools, which allow us to modify genes more precisely and easily. In the future, these technologies will continue to improve in specificity, efficiency, and delivery methods. Research priorities include developing more accurate delivery vehicles, reducing off-target effects, and improving editing accuracy by better controlling editing results. Nucleic acid guidance systems are likely to remain central to genome editing because they are easy to program and adapt. However, editing may shift to more ephemeral methods to reduce the risk of long-term genetic changes or lower immune responses. Advances in artificial intelligence (AI) will help us simulate complex editing scenarios, predict editing outcomes, and design safer editors, accelerating the development of safe treatments.
但我们也不能对基因编辑技术完全的乐观,伦理和社会问题,特别是关于人类生殖细胞编辑的争议,仍然是讨论的重点。虽然体细胞编辑已成为现实,但生殖细胞编辑的治疗性和非治疗性应用,以及其对人类基因组的可遗传改变,引发了深刻的伦理问题。目前的研究表明,CRISPR技术在生殖细胞编辑方面还不够安全或有效,且其治疗作用有限。因此,国际社会迫切需要就基因组编辑的治理和负责任的管理达成共识,尤其是在技术快速发展和广泛应用的情况下。
But we can't be completely optimistic about gene editing technology, ethical and social issues, especially the controversy over human germline editing, remain a focus of discussion. While somatic cell editing is a reality, the therapeutic and non-therapeutic applications of germline editing, as well as its heritable changes to the human genome, raise profound ethical questions. Current studies have shown that CRISPR technology is not safe or effective enough for germline editing, and its therapeutic benefits are limited. Therefore, there is an urgent need for the international community to reach a consensus on the governance and responsible management of genome editing, especially as the technology is rapidly evolving and widely used.
基因编辑的故事,就是科学和现实博弈的一个缩影。它既不是包治百病的 “万能神药”,也不是一吹就破的 “资本泡沫”。每次有突破的时候,我们固然要欢呼,但也得清醒地认识到:疗法上市可不等于问题就解决了,长期安全性验证、可及性提升、伦理框架完善,这些都是漫漫长路。投资也得理性,生物医药可没有 “短平快” 的好事,押注前沿技术就得有容忍失败和等待的耐心。
就像 CRISPR 的发现者杜德纳说的:“我们正在书写生命科学的新篇章,但每一页都需要严谨与责任。” 让我们一边满怀期待,一边敬畏未知,静静等待基因编辑带给我们更多的惊喜吧。